Autophagy is a cellular process that plays a critical role in maintaining a healthy environment for the cell to survive. It does so by degrading and recycling damaged or unnecessary components of the cell.
Autophagy is part of the cellular events that appear under normal conditions. But it can be overactivated when stress conditions have challenged the cell integrity. The source of the stress could be internal, such as nutrient deprivation or oxidative stress caused by a damaged part of the cell. Or it could come from external sources, like pathogen attacks.
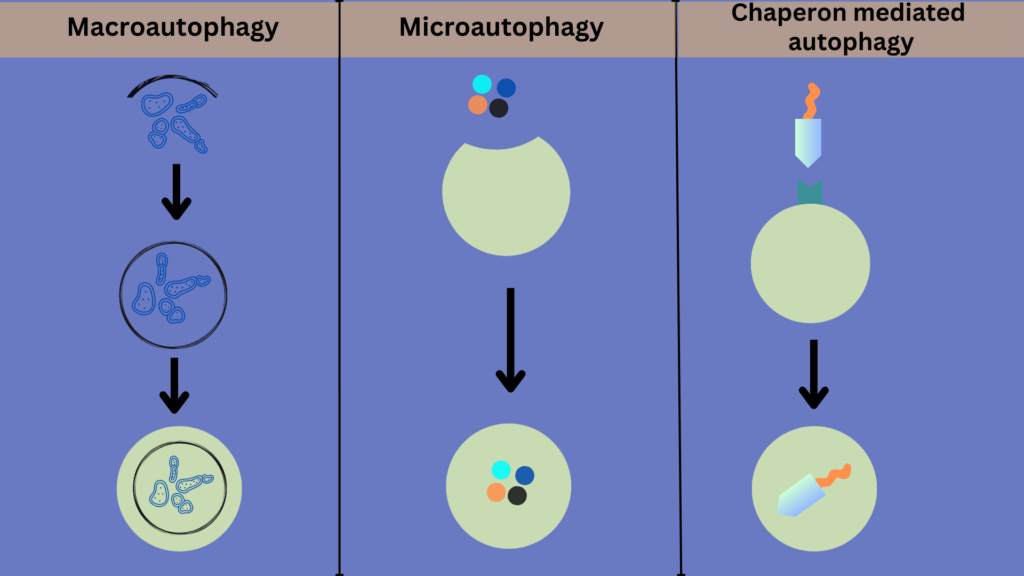
Researchers have divided the autophagy process into three main forms. This division is based on the type of components to be degraded and the mechanism used for their degradation.
This article will explore these different forms of autophagy and their mechanisms.
Let’s get started.
1. Macroautophagy
This is the most well-known form of autophagy. It targets a wide range of cellular components for degradation, ranging from minor parts like proteins to more prominent structures such as whole organelles (like mitochondria). It also deals with the elimination of intracellular pathogens.
The process of macroautophagy begins with forming a phagophore, a cup-shaped structure that originates mainly from the network of membranes known as the endoplasmic reticulum. The phagophore is formed by the assembly of a complex of proteins called the autophagy-initiating complex. This complex is activated in response to nutrient deprivation, cellular stress, or other stimuli.
Once the phagophore is formed, it elongates and expands to form an autophagosome. The autophagosome encloses the targeted cellular components. This process involves the recruitment of additional autophagy-related (ATG) proteins. The autophagosome then fuses with a lysosome, an organelle that acts as a waste disposal unit of the cell. This fusion, mediated by proteins present on the lysosomal membrane, delivers enclosed components into the lysosomal compartment. Inside the lysosome, they are degraded by the actions of enzymes.
The products produced due to degradation are then released into the cytoplasm and re-used to generate energy or as building blocks for synthesizing new cellular components.
2. Microautophagy
Microautophagy is a type of autophagy in which the lysosome directly engulfs the cellular components for degradation. These components could be organelles or proteins; in most cases, it could be a combination of both.
Microautophagy differs from macroautophagy in that the cellular material to be degraded is not sequestered by a double-membrane structure but rather by the direct invagination of the lysosomal membrane. In other words, microautophagy is a more direct form of autophagy.
It is believed that specific proteins regulate microautophagy, although the identity of these proteins is still being investigated. Recently, researchers have identified a set of conserved proteins that play a role in microautophagy, including the ESCRT-III complex and the chaperone protein Hsc70.
3. Chaperon-mediated autophagy
Chaperon-mediated autophagy is a specialized form of autophagy that degrades a specific set of cytosolic proteins. It is different from the above-mentioned two forms of autophagy in that it only targets proteins for degradation, not organelles. Researchers think that this process can degrade at least 30% of cellular proteins.
Many different proteins regulate chaperon-mediated autophagy, but the two major protein players are HSC70 and LAMP-2A. In fact, the whole process is kind of rely on these two proteins. In this process, the HSC70 (which stands for heat shock-cognate chaperone 70 Kda) recognizes the target protein that needs to be degraded and then binds to it. The target protein carries a specific amino acid sequence that serves as the binding site for HSC70. This binding complex then interacts with the lysosomal-associated membrane protein 2A (LAMP-2A), which drives the transport of the target protein to the lysosome. Once the complex reaches the lysosome, the HSC70 protein delivers the targeted protein to the lysosome’s enzymes for degradation.
Chaperon-mediated autophagy is tightly regulated to ensure proper protein turnover and prevent cellular damage. Its regulation occurs at multiple levels and is activated in response to stresses caused by the accumulation of dysfunctional proteins or starvation.
Importance of autophagy
Autophagy plays a crucial role in maintaining a healthy cellular environment by ensuring the proper turnover of cellular components. It is particularly important for degrading long-lived and defective proteins and organelles. If not removed, these structures accumulate in cells over time and can cause various diseases, including neurodegenerative disorders, cancer, and metabolic disorders.
Additionally, autophagy plays a role in response to stress, and this helps the cell to adapt and survive under adverse conditions. For example, in response to oxidative stress, autophagy is activated to remove damaged proteins and organelles, thereby preventing further damage to cellular components.
Related article: Autophagy is amazing – Here’s what research says about its health benefits.