Autophagy is a natural process within the body to remove damaged or unnecessary components and recycle them. This process is crucial to our overall health and well-being. It even plays a vital role in preventing certain diseases.
So how does autophagy work, exactly?
This step-by-step guide will explore the mechanisms behind this critical cellular process. It will discuss what cellular components are degraded by autophagy. Further, it will briefly answer some of the most frequently asked questions about autophagy.
What is autophagy?
The word autophagy comes from the Greek words “auto” meaning “self”, and “phagy” meaning “eating.” Simply put, it is the journey through which a cell degrades its components that are not required. However, it is more complex than it sounds. This journey required several steps and protein complexes that form along the way to facilitate the delivery of target cellular components to their final destination for degradation and recycling.
For the sake of simplicity, researchers have divided the whole process into the following four main steps:
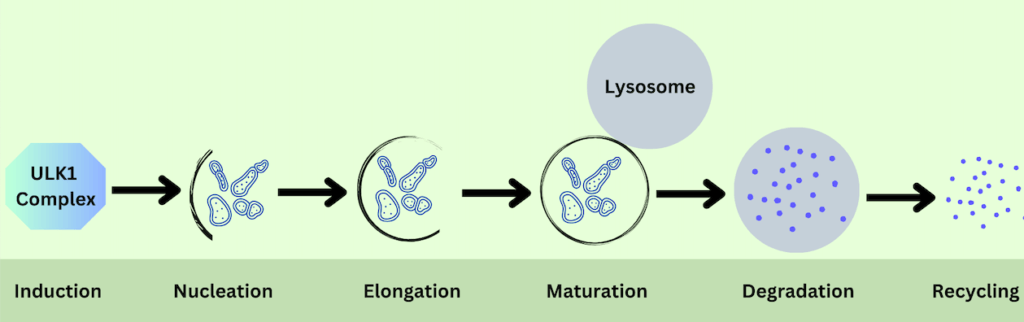
1) Induction
Autophagy is initiated when the cell senses that it needs to eliminate specific components. In this step, a protein complex called the ULK1 complex activates and begins the initiation process. This complex is usually inhibited by mTOR (mechanistic target of rapamycin), a well-known protein that acts as a nutrient sensor within the cell.
The mTOR plays a crucial role in regulating cellular metabolism and detecting nutrient levels. When nutrients are sufficient, mTOR is active and suppresses autophagy initiation. However, when nutrients are limited, mTOR is inhibited, and autophagy is initiated. In other words, mTOR is a negative regulator of autophagy.
The ULK1 complex comprises several proteins, including ULK1 itself, FIP200, ATG13, and ATG101. These proteins work together and facilitate the activation of other proteins required for downstream autophagy processes.
2) Autophagosome formation and maturation
Once autophagy is initiated, the next step is the formation of an isolation membrane that begins to form around the targeted cellular components. This double-membrane structure is called the autophagosome. The formation of autophagosome is a central event in autophagy. Its formation and maturation require the following steps:
i) Nucleation: Several proteins interact and build up the VPS34 complex during the nucleation step. The leading players of this complex are Beclin-1, VPS34, and VPS15. These proteins work together to begin the formation of the autophagosome.
Initially, the autophagosome looks like a cup-shaped membrane sac known as the phagophore. While the exact origin of this membrane is still under debate, most researchers agree that it originates from the endoplasmic reticulum, a network of membranous sheets and tubules inside the cell.
ii) Elongation: The newly formed phagophore then elongates around the targeted cellular component and engulfs it. A two-step reaction system regulates the elongation process. In the first step, the three proteins, namely ATG5, ATG12, and ATG16L, combine to form a ternary complex on the surface of the phagophore. The ATG7 and ATG9 proteins mediate this combination.
The presence of the ternary complex on the phagophore facilitates the second reaction step. During this step, a well-known autophagy protein, LC3, attaches to the phagophore membrane. The attachment of LC3 to the phagophore membrane is a critical step in autophagy, and researchers often use this step to access the autophagy process. And for that reason, the LC3 protein is widely used as an autophagy marker.
iii) Maturation: After being closed and fully formed, the autophagosome progresses toward the maturation phase and starts fusing with the lysosome, a membrane-bound organelle involved in digestion and waste removal inside the cell.
Researchers are trying to understand how the elongated phagophore turns into a fully formed autophagosome. They observed that most of the proteins involved in autophagosome formation dissociate from its surface while the LC3 remains associated until after its fusion with the lysosome. The main proteins involved in this fusion event include Rab, SNAREs, and LAMP1.
In most cases, the matured autophagosome combines with a sac-like structure (the endosome) to form an intermediate organelle before it fuses with the lysosome. This intermediate organelle is called the amphisome. The formation of amphisome is essential for the smooth delivery of autophagosomal contents into the lysosome.
3) Degradation
The fusion of the autophagosome with the lysosome makes it possible for the autophagosome to deliver its contents (the targeted cellular components) into the lysosomal compartment. Here, several enzymes start the degradation process. These enzymes are collectively termed hydrolases. Hydrolases break down cellular components and convert them into their basic building blocks, such as amino acids, fatty acids, and sugars.
4) Recycling
The final step in autophagy is recycling. In this step, the products generated by the lysosomal enzymes are transported out of the lysosome and released into the cytoplasmic compartment of the cell. Once released, the cell uses them for energy production processes like glycolysis and the citric acid cycle. Alternatively, these products can build new cellular components, such as proteins, lipids, or nucleic acids. The recycling phase marks the termination of the autophagy process.
What cellular components are degraded by autophagy?
Autophagy targets a wide range of cellular components for degradation. Some of these components are the following:
- Damaged or malfunctioning organelles such as mitochondria, peroxisomes, and endoplasmic reticulum.
- Misfolded or aggregated proteins that the cell cannot properly process.
- Excess or unnecessary proteins that are no longer needed by the cell.
- Intracellular pathogens such as viruses and bacteria that have been engulfed by the cell.
- Lipids and other molecules that have been stored in specialized cellular compartments.
Notably, autophagy is not the only degradative system inside the cell. There also exists another one called the proteasome system. However, unlike autophagy, the proteasome system only targets proteins for degradation.
Five frequently asked questions about autophagy!
1. What are the main benefits of autophagy?
Autophagy has been linked to many health benefits. It improves cellular health and longevity and may protect against age-related diseases. It can boost immune function and improve metabolic processes.
2. How can I activate autophagy?
Autophagy can be activated through lifestyle interventions such as intermittent fasting, exercise, caloric restriction, adequate sleep, and certain foods and supplements.
3. Does autophagy have any adverse side effects?
Autophagy is a natural cellular process and should not have any adverse side effects. However, certain interventions used to activate autophagy, such as extreme caloric restriction, can have negative consequences if not done correctly.
4. Can autophagy prevent cancer?
Autophagy’s role in cancer is complex and can vary depending on the specific condition. In some cases, autophagy can act as a tumor suppressor and prevent the body cells from initiating and progressing the tumor process. In other cases, autophagy can promote cancer by providing nutrients to tumor cells and helping them survive under stressful conditions.
5. Can anyone activate autophagy?
Yes, anyone can activate autophagy through lifestyle interventions. However, consulting a healthcare professional before starting any new dietary or exercise regimen is essential.